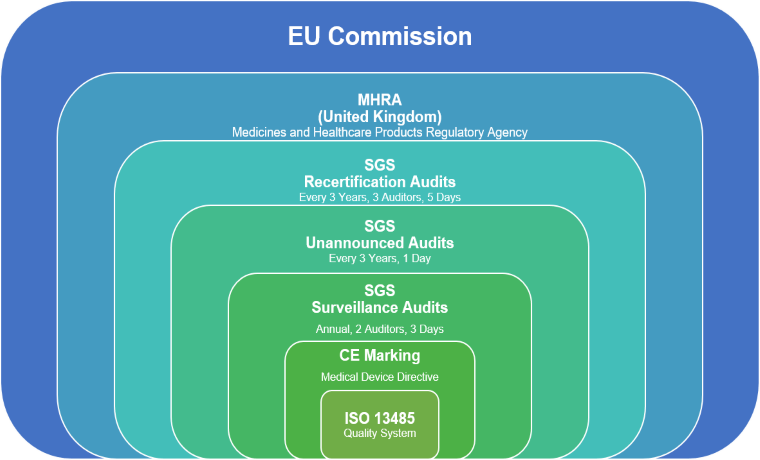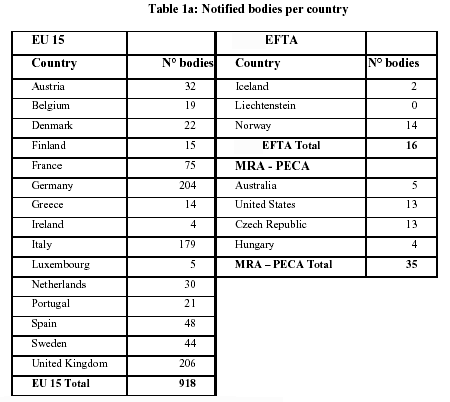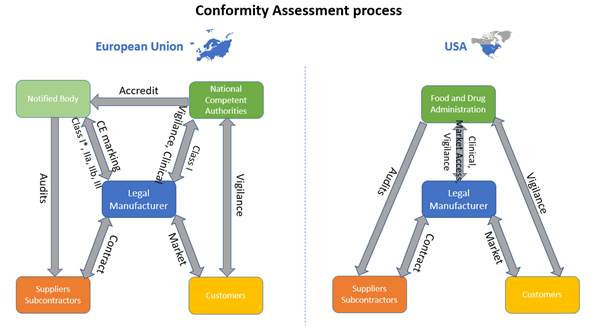
What are the principal differences between the conformity assessment process of a medical device in the USA and in the European Union? - Kvalito

Member state of the European Union ATEX directive CE marking, 60 minutes, european Union, safety, notified Body png | Klipartz

CE Mark for All Directives by EU Notified Bodies at Rs 20000/certificate in Palghar | ID: 4438471733

Unannounced notified body visits recommendation imminent – amend your contracts and procedures now! | medicaldeviceslegal

Competent Authority, Notified Body, ISO Registrar: How Each Role Functions in the Medical Device Industry